The last week I’ve been working on the final report of a project I was running over the last three years. Mostly this is mind-numbingly boring stuff, but now a one-page popular science summary should be included. Since I had to write it I can just as well put it to use in the blog. If you have any ideas for improvement, please tell me. Unfortunately, since most people have no clue about ion transfer, and maybe for good reason, about 80% is introduction and only in the end I touch upon what we actually did…
Oil and water don’t mix. But when oil and water are brought together chemicals dissolved in the oil might migrate across the oil-water interface into the water. And of course chemicals in the water can move into the oil. Transfer of chemicals across the interface between liquids that don’t mix is important in many biological processes like photosynthesis in plants or transport across the cell membrane. It has also been suggested that it can make chemical synthesis more efficient if the product could be transferred to a different liquid to reduce the need for purification after the reaction.
How much of the compound that is transferred when the liquids meet depends on the transfer energy. This is a measure on how much energy it costs to move a particle from one liquid to another. The value of the transfer energy depends on the concentration of the compound, how hydrophilic (water loving) or hydrophobic it is and the properties of the liquids. If the transferred species is an ion, that is a charged molecule, the transfer energy also depends on the electric potential difference between the liquids. The potential difference can be controlled by putting electrodes into the liquids and applying an electric potential. By measuring the ion current researchers can determine at which potential the ions are transferred. This information can be used either to determine the hydrophilic or hydrophobic nature of the ions or, if this is already known, to identify and measure the concentration of the ions.
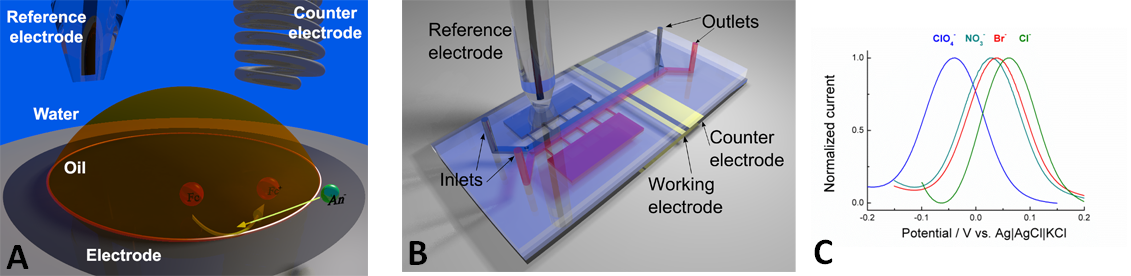
A) Sketch of the ion transfer setup in a droplet system. B) The ion transfer system in a microfluidic system with two parallel streams. C) The transfer potential measured in the microfluidic system for different anions, from ClO4- which is hydrophobic to the very hydrophilic Cl-.
In our experiments we have used a different method for controlling the potential difference between the liquids. If a redox reaction, i.e. a reaction where a chemical either takes up electrons (reduction) or gives away electrons (oxidation), happens at an electrode in one of the liquids it will lead to an imbalance in the charge between the liquids. It is important that the chemical that undergoes the redox reaction is soluble in either the water or in the oil, but not in both liquids. Fortunately, several such chemicals are available. In our case ferrocene was oxidised in the oil. This means that there is an excess of positive charge in the oil. That in turn changes the transfer energy, and to compensate anions (ions with a negative charge) will be transferred from the water to the oil (Fig. A). Again, at which potential depends on the nature of the ion (Fig. C). The standard way of doing this is with an oil drop on an electrode inserted into an aqueous electrolyte. A model for calculating the transfer potential was developed for this system about 15 years ago.
One of the most important developments in chemistry over the last decades has been the development of microfluidic systems for analysis and synthesis. This has led to the development of lab-on-a-chip devices where reactions that previously took a whole lab now can be done on a chip, much faster and with lower consumption of chemicals.
We have used a microfluidic system for study of ion transfer where the oil and water are flowing parallel through the channel (Fig B) and ion from the water phase is transferred to the oil. In our experiments we managed to show that the model developed for the droplet system is also valid in this system. Importantly, even though the ion current has a complicated dependence on the flow rate, with the current first decreasing and later increasing with increasing flow rate, we found that the potential is independent of this parameter. This is a crucial point, because if the transfer potential was dependent on the rate of flow it would make analysis of the data much more difficult. These results are an important step towards introducing microfluidics as an modern tool in studies of ion transfer and in constructing innovative devices for diagnostic purposes.